Properly distinguishing between true infections and carriers is crucial to protecting patients from unnecessary treatments and supports antimicrobial stewardship efforts. Rapid and accurate identification of toxigenic C. difficile allows for appropriate treatment when needed, supporting patients as they recover their health, reconnect with loved ones, and reclaim their quality of life.
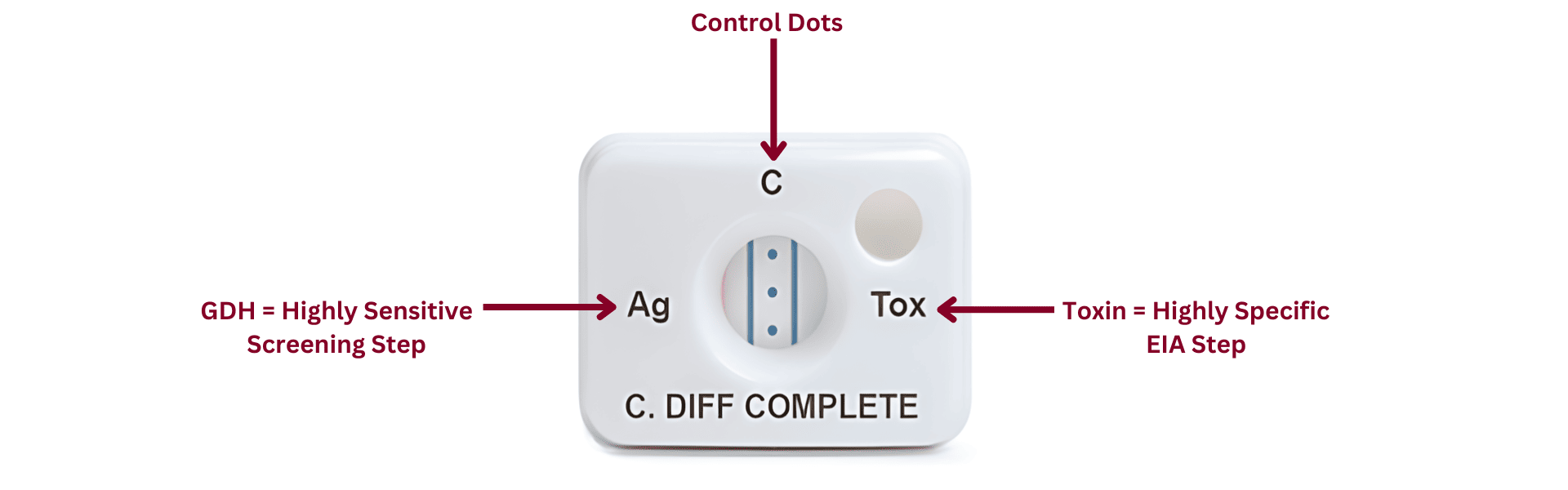
C. difficile diagnostic testing options
A nuanced understanding of the available tests can help healthcare providers deliver accurate diagnoses, protect patients, and provide the best possible care. By leveraging these diagnostic tools thoughtfully using a multi-step algorithmic approach, healthcare providers can ensure accurate and timely diagnosis for better patient outcomes. This approach not only addresses the immediate medical needs of patients but also allows for a healthcare experience that prioritizes appropriate treatment, recovery, and well-being.