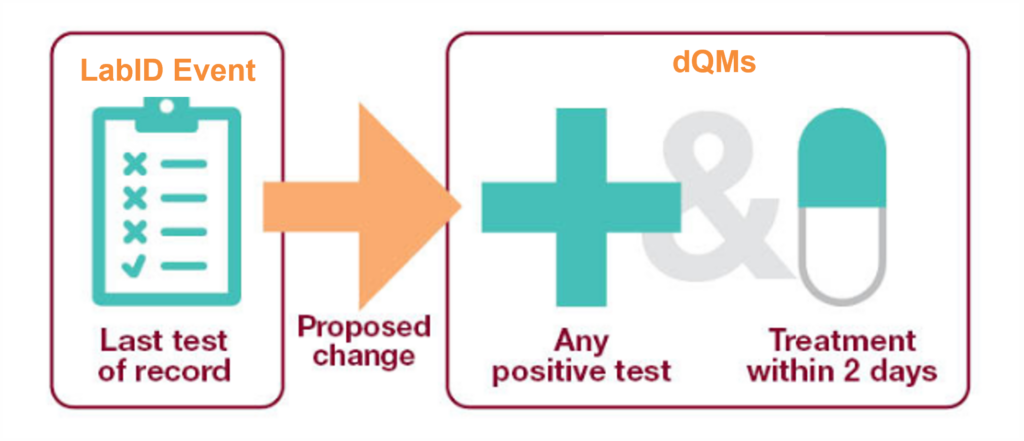
What do the guidelines say?
To ensure patients receive appropriate diagnosis and treatment for C. difficile infection, the 2021 American College of Gastroenterology (ACG) Clinical Guidelines2 and the 2018 Infectious Diseases Society of America (IDSA) / Society for Healthcare Epidemiology of America (SHEA) Guidelines3 emphasize the importance of a multistep algorithmic approach comprised of:
A Sensitive Screening Test:
GDH or Nucleic Acid Amplification Test (NAAT) effectively rules out difficile infection (CDI) with high sensitivity. Studies confirm equivalent clinical sensitivity of both methods in ruling out CDI.4-7
A Specific Toxin Test: Toxin enzyme immunoassay (EIA) tests offer excellent clinical specificity, ensuring that diagnoses align with actual disease states.