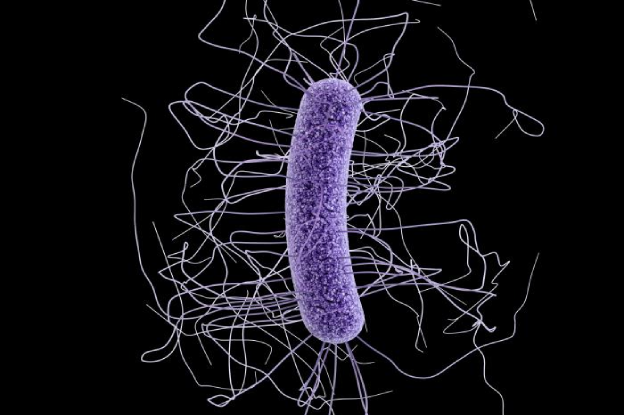
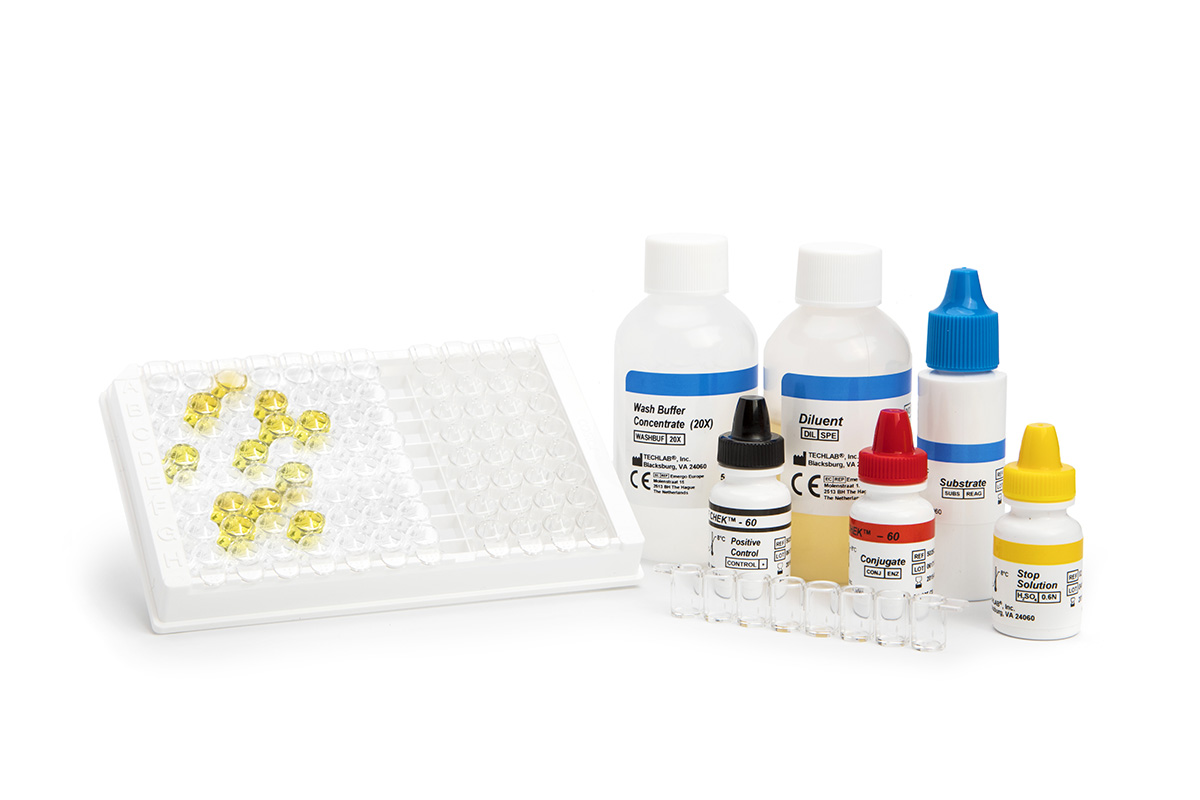
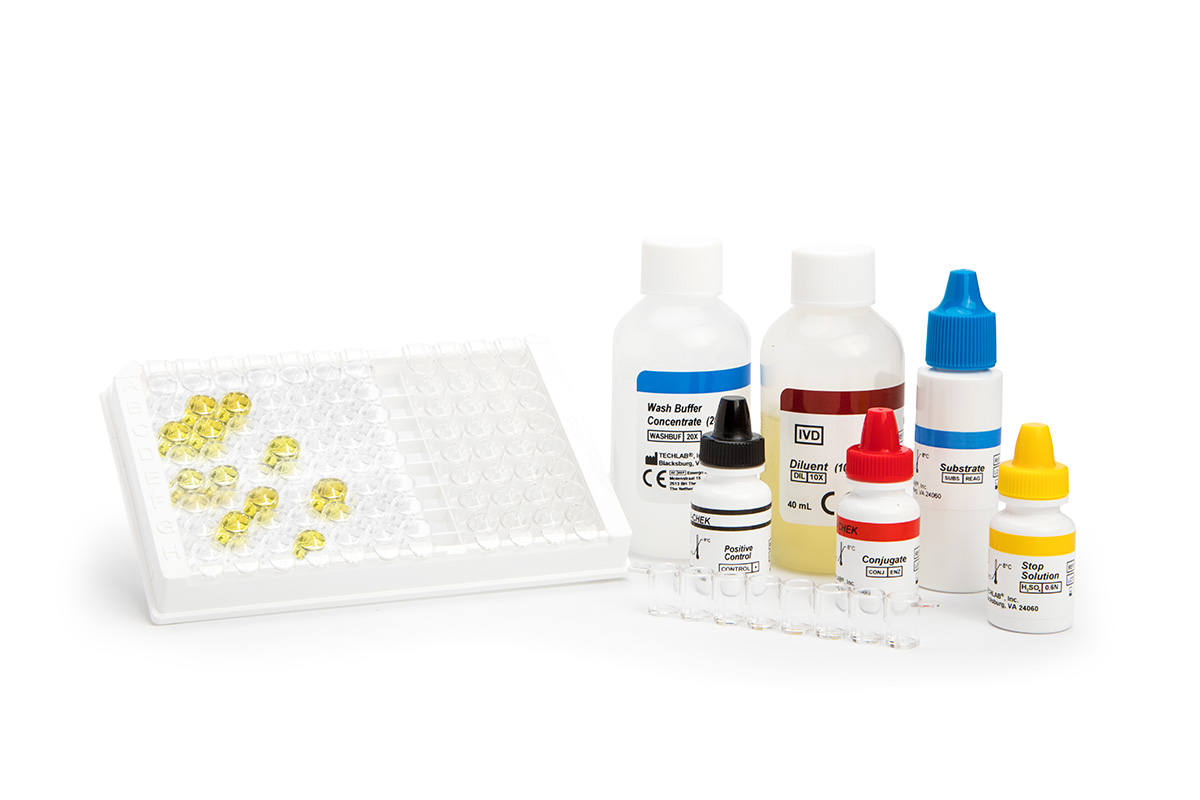
C. difficile infection usually occurrs when the normal microflora of the intestinal tract is altered or killed by antibiotics. Once C. difficile growth begins, toxins A and B are produced, causing diarrhea and colitis.
Toxigenic strains of C. difficile carry the genes encoding the toxins. The disease results from the toxins that the organism produces. C. difficile produces two main toxins, toxin A and toxin B. Toxin A is a weakly cytotoxic enterotoxin. C. difficile also produces toxin B, which is a cytotoxin. Toxin B can be detected by the tissue culture assay. Toxigenic C. difficile strains produce both toxins or only toxin B.
The disease can be treated with antibiotics, but relapses are common.
Read more about The Importance of C. difficile Diagnostics
Read more about a complete stand-alone 2-step C. difficile algorithm in one test