- Home
- E. HISTOLYTICA QUIK CHEK™
[col_23]
Entamoeba histolytica is an intestinal parasite estimated to infect 50 million people world-wide. Infected individuals can develop bloody diarrhoea, colitis and liver abscesses and up to 100,000 deaths from amebiasis are estimated annually.1 E. histolytica disease has also been diagnosed in 12% of travellers returning from the developing world with acute diarrhea.2
[image source=”https://www.techlab.com/wp-content/uploads/2014/09/e-histo-product.jpg” alt=”E. HISTOLYTICA QUIK CHEK™” align=”right” border=”true”]
The First 30-minute E. histo Diagnostic
The E. HISTOLYTICA QUIK CHEK™ test is a rapid membrane enzyme immunoassay for the simultaneous qualitative detection and differentiation of the pathogenic Entamoeba histolytica and the non-pathogenic E. dispar. It is intended for use with human fecal specimens from patients with gastrointestinal symptoms to aid in the diagnosis of E. Histolytica parasite. The test results should be considered in conjunction with the patient history.
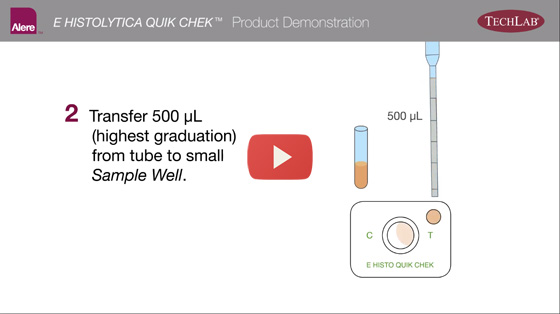
How it Works
The sample is added to a tube containing a mixture of Diluent and Conjugate. The diluted sample-conjugate mixture is added to the Sample Well and the device is allowed to incubate at room temperature for 15 minutes. During the incubation, parasite antigens in the sample bind to the antibody-peroxidase conjugates. The antigen-antibody-conjugate complexes migrate through a filter pad to a membrane where they are captured by the immobilized antibodies in the test lines. The Reaction Window is subsequently washed with Wash Buffer, followed by the addition of Substrate and a 10-minute incubation period.
The E. Histolytica reaction is examined visually for the appearance of a vertical blue line on the right side of the Reaction Window. A blue line indicates a positive test.
This brief presentation is intended for demonstration use only, and not as a substitute for the product instructions. Please refer to the E. HISTOLYTICA QUIK CHEK™ package insert accompanying each test kit.
References
1. Buss, S. et al. (2008) Comparison of Two Immunoassays for Detection of Entamoeba histolytica. J. Clin. Micro. Vol 46, No. 8, pp.2778-2779.
2. Freedman, D.O. et al. (2006) Spectrum of Disease and Relation to Place of Exposure among Ill Returned Travelers. N. Engl. J. Med. 354;2.
[/col_23]
[col_14_last]
Features
[arrowlist]
- Distinguishes between E. Histolytica and the non-pathogenic E. dispar
- Results in 30 minutes without instrumentation
- Offers alternative to labor-intensive ELISAs and PCRs
- Simple and clear interpretation of results
[/arrowlist]
This product is only available outside the United States. It is available exclusively through our global distributor, Alere.
[/col_14_last]