TESTING FORMATS
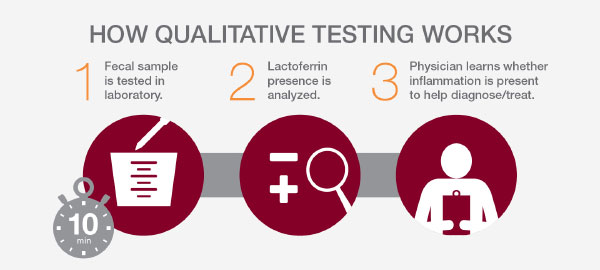
Qualitative testing
LACTOFERRIN CHEK® and LACTOFERRIN EZ VUE® are in vitro qualitative assays that measure elevated levels of fecal lactoferrin in stool specimens. Both provide a yes-or-no result for the presence of intestinal inflammation.
Qualitative lactoferrin testing provides a positive or negative result for elevated levels of fecal lactoferrin. It can rapidly and non-invasively:
- Distinguish between patients with IBS and active IBD.
- Identify IBD patients with active inflammation.
- Provide convenient results for smaller clinics with appropriately-licensed in-house labs.
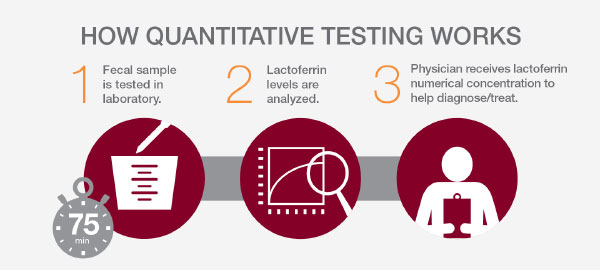
Quantitative testing
LACTOFERRIN SCAN® is a quantitative ELISA that accurately and quantitatively measures the levels of lactoferrin in a stool sample. The test result can be used to help distinguish patients with IBD who will have elevated levels of fecal lactoferrin from those with IBS.
Quantitative lactoferrin testing helps to quickly and non-invasively:
- Distinguish between patients with IBS and active IBD.
- Assess inflammation levels during IBD treatment to help direct treatment and to aid in predicting relapse.
Assess C. difficile disease severity and guide treatment.
LACTOFERRIN SCAN®
A quantitative ELISA that accurately and quantitatively measures the levels of fecal lactoferrin in a stool sample. The test result can be used to help distinguish patients with IBD who will have elevated levels of fecal lactoferrin from those with IBS.
Order lactoferrin testing
Both qualitative and quantitative lactoferrin testing can be easily ordered through most laboratories and are covered by most insurance plans.
- QUALITATIVE TESTING: CPT CODE 83630
- QUANTITATIVE TESTING: CPT CODE 83631