- Home
- Diagnostics
- Parasitology
- TRI-COMBO PARASITE SCREEN – T30408
[col_23]
[image source=”https://www.techlab.com/wp-content/uploads/2014/02/t30408.jpg” alt=”TRI-COMBO PARASITE SCREEN” align=”right” border=”true”]
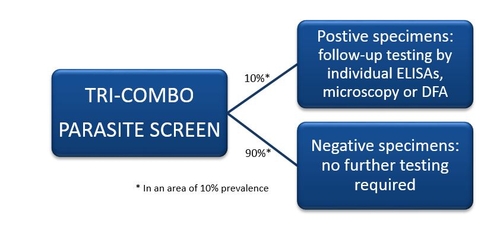
The TRI-COMBO PARASITE SCREEN test is an enzyme immunoassay for the simultaneous qualitative detection of Giardia spp., Cryptosporidium spp. and/or E. histolytica antigen in human fecal specimens. The test is indicated for use as a screen for fecal specimens from patients with gastrointestinal illness including dysentery to aid in the diagnosis of enteric infection resulting from giardiasis, cryptosporidiosis or amebiasis. The TRI-COMBO PARASITE SCREEN test serves as a screen to rule out negative specimens and reduce the number of specimens that require additional follow-up testing.
The TRI-COMBO PARASITE SCREEN test uses monoclonal and polyclonal antibodies to cell-surface antigens of Giardia, Cryptosporidium and E. histolytica. The microassay plate in the kit contains immobilized monoclonal antibodies against the antigens, and the Conjugate contains polyclonal antibodies against the antigens.
In the assay, an aliquot of a diluted fecal specimen is transferred to a microassay well. The immobilized monoclonal antibodies bind the Giardia, Cryptosporidium and/or E. histolytica antigens if they are present. Conjugate is then added and binds to the antigen-antibody complex. Any unbound materials are removed during the washing steps. Following the addition of Substrate, a color develops in the presence of enzyme-antibody-antigen complexes that formed in the presence of antigens and Conjugate.
| Prevalence rate | Using the TRI-COMBO PARASITE SCREEN | Without the TRI-COMBO PARASITE SCREEN |
| 10% | 130 total tests (100 on TC + 30 individual tests) | 300 individual tests run |
| 20% | 160 total tests (100 on TC + 60 individual tests) | 300 individual tests run |
| 50% | 250 total tests (100 on TC + 150 individual tests) | 300 individual tests run |
With microscopy, potentially 300 samples requiring individual analysis
(if testing 3 samples per patient as recommended)
Further Information
Package Insert (PDF) Issued 04/14
CPT Codes (PDF) Issued 02/2023
Declaration of Conformity (PDF) Issued 9/14
Publication
Multisite Performance Evaluation of an Enzyme-linked Immunosorbent Assay for Detection of Giardia, Cryptosporidium, and Entamoeba histolytica Antigens in Human Stool
Christy NC, Hencke JD, Escueta-De Cadiz A, Nazib F, von Thien H, Yagita K, Ligaba S, Haque R, Nozaki T, Tannich E, Herbein JF, Petri WA Jr. J, Clin Microbiol May 2012 50(5):1762-3. Epub 2012 Feb 29
Publication
Diagnosis of Multiple Enteric Protozoan Infections by Enzyme-linked Immunosorbent Assay in the Guatemalan Highlands
den Hartog J, Rosenbaum L, Wood Z, Burt D, Petri WA Jr. Am J, Trop Med Hyg. 2013 Jan;88(1):167-71. doi: 10.4269/ajtmh.2012.12-0142. Epub 2012 Nov 26
Poster Presentation (PDF)
Assessment of a New Parasitology Screening Diagnostic ELISA for the Detection of Antigens of Giardia spp., Cryptosporidium spp., and Entamoeba histolytica in Fecal Specimens
American Society of Tropical Medicine and Hygiene, December 2011, Philadelphia, PA
Poster Presentation (PDF)
Evaluation of a Diagnostic Screening ELISA for the Detection of Giardia spp., Cryptosporidium spp. and E. histolytica in Human Fecal Specimens
American Society of Tropical Medicine and Hygiene, December 2011, Philadelphia, PA
Poster Presentation (PDF)
Simultaneous Detection of Entamoeba histolytica, Cryptosporidium spp., and Giardia lamblia in Fecal Samples Using a Single Enzyme Immunoassay
Infectious Diseases Society of America, October 2008, Washington, DC
[/col_23]
[col_14_last]
Features
[arrowlist]
- Results in less than 2 hours
- Simple procedure
- Highly specific
- Ideal screening test for the three most common parasitic causes of diarrhea
[/arrowlist]
This product is only available outside the United States. It is available exclusively through our global distributor, Alere.
[/col_14_last]